
Ensovibep / Sars-Cov-2: COVID
Ensovibep is a COVID-19 antiviral therapeutic candidate designed specifically to inactivate SARS-CoV-2 – the virus that causes COVID-19 – with extremely high potency that is preserved against novel variants. In January 2022, we announced positive topline results from our Phase 2 global clinical study of ensovibep in acute COVID-19 ambulatory patients showing approximately 80 percent reduction of the of combined risk of hospitalization, emergency room visits, or death compared to placebo, regardless of vaccination status. It was shown to be safe and well-tolerated. Comprehensive in vitro studies of ensovibep demonstrate its high inhibitory potency against all COVID-19 variants of concern, including Omicron, as of December 2021. Novartis has informed Molecular Partners of its intent to in-license ensovibep and is expected to lead late-stage development and commercialization.
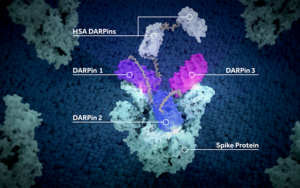 Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.
Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.
Mechanism of Action
Need
COVID-19 represents the biggest disease burden in the world today through its impact on healthcare, society and economies. A multi-solution strategy is needed to combat the pandemic and the need for antiviral treatments to complement global vaccination efforts has never been greater. As disease transmission continues through pockets of unvaccinated populations, in patients with compromised immune systems and co-morbidities, and as variants continue to emerge, millions of patients around the world are in need of differentiated therapies that are specifically designed to combat COVID-19 in all its forms.
Rationale
The DARPin platform is designed to be used to rapidly generate diverse, multifunctional drug candidates, capable of binding to multiple targets at once. As a tri-specific candidate with cooperative binding, ensovibep’s unique pan-variant design has allowed it to retain full potency against all known variants of concern including the Omicron variant in comprehensive preclinical studies conducted to-date.
Solution
Ensovibep is a unique tri-specific candidate designed to preserve potency against viral variance. It exhibits among the most potent viral inhibition against all variants of concern reported to date. In the EMPATHY Phase 2 global clinical trial, ensovibep demonstrated approximately an 80 percent reduction of combined risk of hospitalization, emergency room visits or death in non-hospitalized patients compared to placebo regardless of vaccination status. It was shown to be safe and well-tolerated. If approved or authorized, ensovibep will be the first multi-specific antiviral molecule for the treatment of COVID-19.
Mechanism of Action



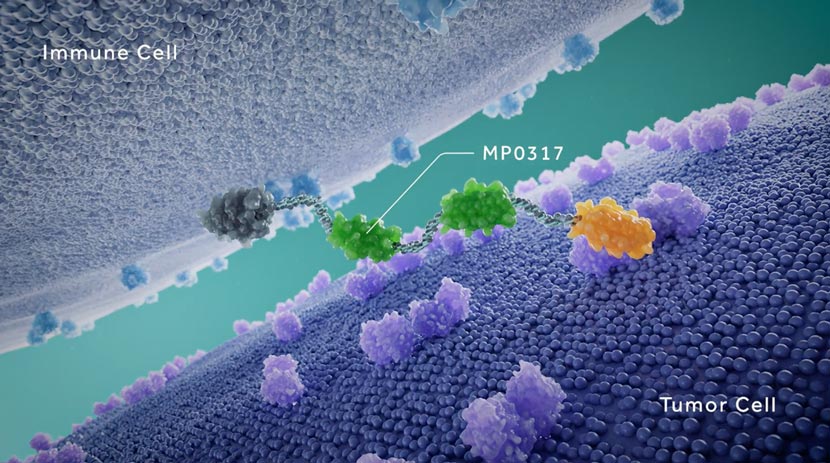
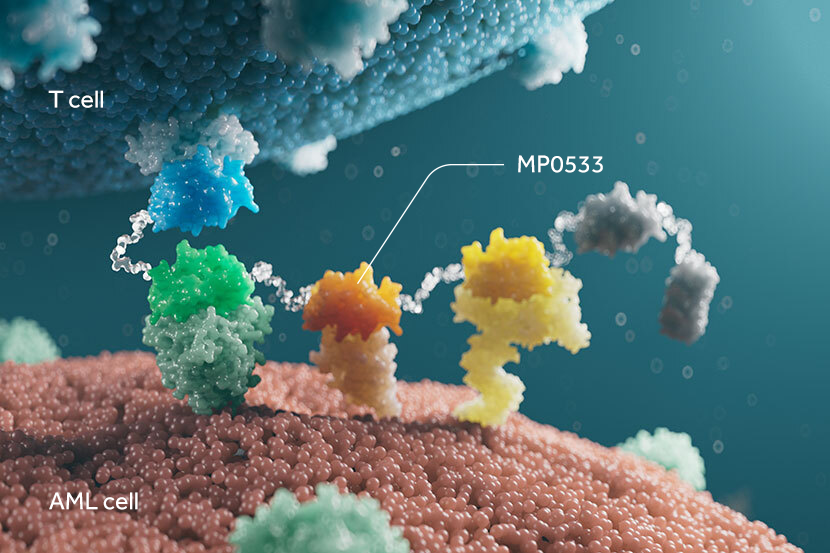
 Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.
Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.