Partnering
Uniting therapeutic innovation
with global capabilities
At Molecular Partners, we believe that strong collaborations are an essential component to realize the full potential of DARPin therapeutics for patients. We aim to collaborate with partners that share our drive and commitment to accelerate the discovery and development of breakthrough therapies for patients.
Our partnerships are tailored to the DARPin therapeutic and mechanism of action in question, the medical problem and indication, as well as the objectives and potential synergies between us and our partners. We strive for fast and nimble decision-making from idea generation through to ensuring the partnerships function efficiently and at full capacity.
To date, Molecular Partners has received combined upfront and milestone payments of approximately CHF 450 million in connection with agreements with our partners.
In January 2024, Molecular Partners entered into a co-development agreement with Orano Med, a pioneer in targeted alpha therapy, to develop novel Radio-DARPin Therapies (RDTs) that use Orano Med’s 212Pb radioisotope as an alpha particle-emitting payload to selectively kill cancer cells and leverage the unique properties of DARPins as potentially ideal vectors for radioisotopes. The partnership combines Molecular Partners’ leadership in DARPins with Orano Med’s leading expertise and unique capabilities in 212Pb-based targeted alpha therapy (TAT) preclinical and clinical development.
Through the collaboration, the companies will share costs for preclinical and clinical development for four programs, the first of which targets DLL3 (MP0712) and the second of which targets MSLN (MP0726). Molecular Partners holds commercialization rights for both of these programs.
In January 2025, the strategic collaboration was expanded to include the development of an additional six targeted alpha therapeutics candidates, representing a total of ten potential programs between the two companies. Molecular Partners will lead the development of the additional six programs, subject to a royalty arrangement, with an option for Orano Med to move two of the six programs into a 50/50 co-development where Orano Med will hold commercialization rights.
Legacy Partnerships
We have continued to expand our capabilities and those of our DARPin candidates. This is underpinned by our deep clinical experience with DARPin programs, across development stages through to the registrational phase, and informed by development work performed with partners.
In October 2020, we entered into a collaboration with Novartis to develop, manufacture and commercialize our DARPin candidates for COVID-19. Subsequently, Novartis in-licensed ensovibep, a trispecific candidate. Following the positive results of the Phase 2 global EMPATHY clinical study, Novartis exercised its option to in-license ensovibep in January 2022 and Molecular Partners received a milestone payment of CHF 150 million.
As the circumstances of the pandemic evolved, an Emergency Use Authorization (EUA) for ensovibep was withdrawn effective in January 2023. Novartis returned the rights to the ensovibep program to Molecular Partners in January 2024. Clinical work on the ensovibep program ended in 2022.
The program provided an important clinical validation of the application of DARPins in virology.
In December 2021, we entered into a research and collaboration agreement with Novartis to develop, manufacture and commercialize Radio-DARPin Therapies (RDTs) which concluded in March 2025. Molecular Partners received an upfront payment of $20 million.
This collaboration enabled Molecular Partners to launch its own activities in targeted radiopharmaceuticals.

AbbVie and Molecular Partners established a discovery alliance focused on ophthalmology through AbbVie’s subsidiary, Allergan, the initial collaborator beginning in 2011. The key candidate of the collaboration was abicipar, which was developed through to the registrational phase for the treatment of neovascular age-related macular degeneration (nAMD) and Diabetic Macular Edema (DME). The program provided important clinical, drug design and manufacturing learnings that have informed subsequent portfolio development. The program is no longer in active clinical development and the discovery alliance is now terminated.


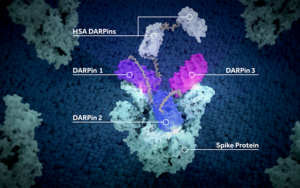 Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.
Ensovibep offers a differentiated approach to treating COVID-19 through a single molecule that can target up to three parts of the SARS-CoV-2 virus simultaneously to neutralize the virus through cooperative binding. This offers potentially broader efficacy and reduced potential for the development of viral drug resistance. All DARPins are produced through a rapid, high-yield microbial fermentation process.